Abstract
Biomarkers for Thrombosis in COVID-19: A Role for High Sensitivity Troponin-I and Immature Platelet Fraction?
Introduction
Coronavirus disease 2019 (COVID-19) is associated with increased risk of thrombosis with both venous and arterial thromboembolism observed. While d-dimer elevation has been shown to be associated with thrombosis, this elevation is present in over 50% of COVID-19 infections demonstrating a clear need for more specific biomarkers of thrombosis in this population. While there are a variety of theories to explain the increased risk for thrombosis: all center on Virchow's triad, specifically hypercoagulability and inflammation. Platelets play a significant role in hypercoagulability. Immature platelets, which are thought to be hyper-reactive, may specifically be associated with thrombosis in COVID-19. It would thus be reasonable to expect immature platelet fraction (IPF) and immature platelet count (IPC) to be predictive biomarkers of thrombosis in this population. Beyond hypercoagulability, High-Sensitivity (HS) Cardiac troponin-I can be a biomarker of inflammation and may also be predictive of thrombotic events in COVID-19. The aim of this study was to evaluate the relationship between IPF, IPC, HS cardiac troponin-I and thrombotic events in COVID-19.
Methods
Using a single center COVID-19 data registry, we extracted all patients with COVID-19 at our single center between May 1, 2020 and January 1, 2021. Patients were stratified into two groups based on thrombotic events during hospitalization, the thrombosis and no thrombosis groups. Biomarker values, including IPF, IPC, platelet counts, d-dimer, and HS cardiac troponin I were extracted. Two-sided Wilcoxon rank test was conducted to test group differences in IPF, IPC, platelet, d-dimer, and HS cardiac troponin-I values.
Results
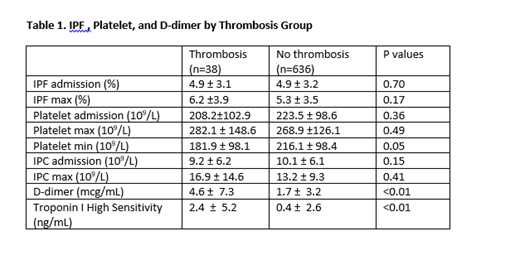
There were no significant differences in measurements of IPF at admission, peak IPF , platelet count at admission, peak platelet count, IPC at admission, and peak IPC between the thrombosis and no thrombosis groups. Minimum platelet count values were significantly lower in the thrombosis group compared to the no thrombosis group. D-dimer and troponin values were significantly higher in the thrombosis group than the no thrombosis group. (Table 1)
Discussion
To our knowledge this is the first study assessing the relationship between IPF, IPC, HS cardiac troponin-I and thrombosis in COVID-19. HS cardiac troponin did appear to be a predictive biomarker for thrombosis in COVID-19. This may be related to vascular inflammation playing a significant role in thrombosis. It may also be secondary to myocardial inflammation associated with severe disease in COVID-19.3 Patients with more severe disease are more prone to thrombosis. Unsurprisingly, our study corroborates evidence in the literature that d-dimer is associated with thrombotic events in COVID-19. On the other hand, IPF and IPC do not appear to be predictive biomarkers for thrombosis in this cohort. This appears consistent with the limited data assessing the relationship between IPF, IPC, and thrombosis outside of COVID-19. This does not dispel the importance of immature platelets in COVID-19, however. IPF and IPC are increased in patients with COVID-19, and our published data indicates they are predictors of COVID-19 severity. However, the relationship between immature platelets and outcomes in acute illness can be complex, as in sepsis, where the trend of IPC is associated with mortality, rather than the initial value. Future studies should delineate the relationship between trends in IPF or IPC and outcomes in COVID-19. Furthermore, it is crucial to define biomarkers of thrombosis and disease severity and mortality in COVID-19 which can potentially guide therapeutic interventions.
No relevant conflicts of interest to declare.